Ask around: how many of your friends have tried to make a sourdough starter… and failed? Probably more than you’d think. If you know me, count me among them. There’s always that one annoying friend for whom it worked on the first try. But for a significant number it didn’t, and they never really knew why. They followed the guide, used the right flour, stuck to the ratios. And still, it failed.
It always seemed strange to me that something humans have done for millennia can feel so fragile. And it made bakers seem almost mythical. Guides you find online or in books offer measurements, timelines, and vague cues. But if you follow them blindly, things often fall apart. And what to do then?
Bakers are truly remarkable, especially when seen through a historical lens. Their deep, intuitive knowledge was originally built through generations of trial and error. Just think of how many people throughout history had to eat bad bread before we figured it out. But what if you don’t have the connections, the experience, or the time to build that intuition? There’s another way to make sense of sourdough: microbiology.
That’s where it clicked for me. Sourdough isn’t guesswork, it’s biology you can learn to read by smell, sight, and touch. In a lab, you’d use flow cytometry, pH meters or DNA sequencing. As a baker, you observe scent, sourness, bubbles, and texture. Different tools for the same goal: understanding the life at work in flour and water.
I believe that basic microbiology builds intuition. It helps us adapt, troubleshoot, and ultimately become better bakers, faster. Here’s my attempt to share that with you.
To understand what’s really happening in your jar, we have to look under the hood and into the biology of fermentation, as sourdough baking is essentially initiating and sustaining a fermentation process.
Fermentation has been used for millennia. The earliest identified use of fermentation was to make alcohol, and archaeology dated that from 7000 to 6600 BCE in China. Is it really a surprise that we might have started with alcohol brewing? However, it’s only recently that chemistry started to make sense of it. We owe the foundational scientific understanding of fermentation to Louis Pasteur in the 19th century.
Before Pasteur, a significant part of the scientific community believed in spontaneous generation. It was the idea that life, like mold on bread, could emerge from non-living matter. Pasteur famously debunked this with his swan-neck flask experiments, showing that microbes arise from other microbes, not spontaneously. His work provided evidence for germ theory of disease and, in doing so, he explained the process of fermentation.
Life without air
Everybody knows what fermentation is: the taste, the smell… But do they really? What, exactly, is fermentation? Louis Pasteur once called it “la vie sans air” (i.e. “life without air”). Fermentation is a metabolic process (one that sustains life by generating energy) that takes place in the absence of oxygen. In it, microbes consume sugars and produce various byproducts such as acids, alcohol, and gases along with small amounts of ATP, the molecule that carries energy within cells.
In yogurt, this process is primarily lactic acid fermentation. In beer and wine, it’s alcoholic fermentation. In sourdough, it’s a mixed fermentation that produces a combination of acids (lactic acid, acetic acid), alcohol, and carbon dioxide gas.
Schematically, there are three main actors in sourdough baking: enzymes, yeasts, and bacteria. We’ll talk about all three. They form a microbial ecosystem with deeply interconnected roles. Enzymes feed both yeasts and bacteria. Yeasts contribute to the dough’s rise and aroma by producing gas and alcohol. Bacteria generate acids that lower the pH. This acidity shapes flavor, inhibits spoilage organisms, but also modulates enzyme and yeast activity. Some bacteria also feed on metabolic byproducts from yeast, and vice versa. As the environment acidifies, strains that tolerate acid are favored, balancing the culture.
As you’ll see throughout this piece, sourdough baking is all about finding balance. The surprising part is how much of that balance will happen naturally, so there’s no need to overthink it. Overthinking it is probably one of the most common mistakes.
Your role as a baker will mostly consist of initiating the process, stepping in before things go off track, and marveling at the life taking shape in a jar.

Fig 1 - Could it be that the Arquilian Galaxy is sourdough?
So what exactly makes this microbial ecosystem thrive? It starts with enzymes.
Enzymes
Simply put, amylase-catalyzed hydrolysis of starch yields fermentable carbohydrates. Wait, don’t run. By the end of this paragraph you’ll understand what that means. First things first: carbohydrates is just the scientific term for sugars, and there are a lot of different kinds.
In sourdough, enzymes play a critical role by breaking down starch (large carbohydrates found in flour) into simpler sugars that yeast and bacteria can consume, much like parents gently cutting their child’s food into bite-sized pieces. Starch molecules are simply too large for these microbes to metabolize directly.
Conveniently, the enzymes required to break down starch are already present in flour. In the wheat plant, these enzymes helped seeds germinate by converting the stored starch in the plant into usable energy. In a way, here with flour, we’re repurposing this mechanism for dough fermentation.
That’s new management, enzymes, now you’re helping us make bread.
There’s a specific name for starch-breaking enzymes – heck, there’s a scientific name for everything. Here, it’s amylases. If you’re wondering why they’re not called starchase, it’s because starch’s scientific name is amylum. The simple sugars amylases produce are known as fermentable sugars because they can be metabolized by microbes during fermentation.
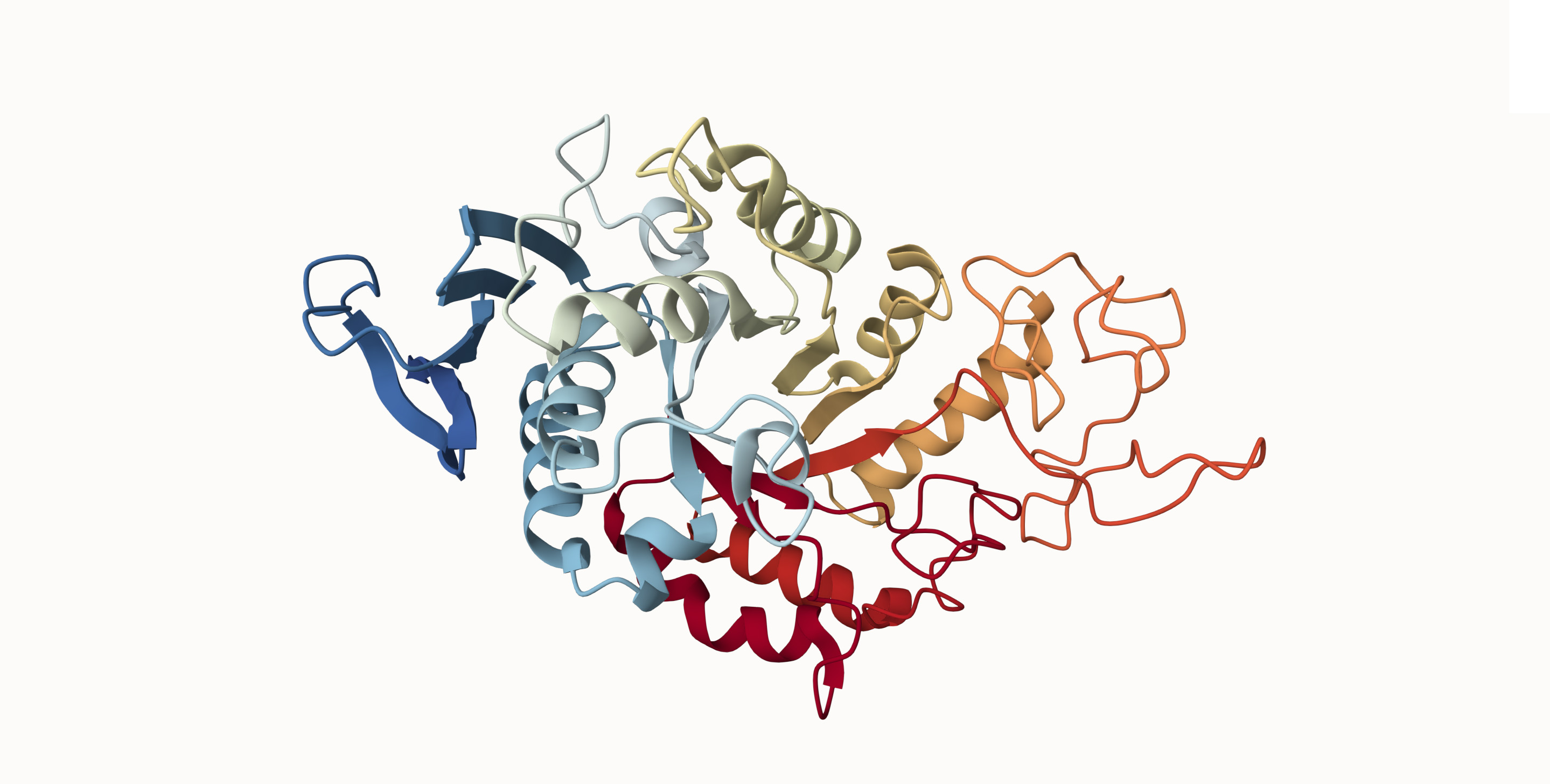
Fig 2 - Crystal and molecular structure of barley α-amylase (PDB ID: 1AMY). Ribbon diagram created in Mol*
Enzymes are just proteins that have a catalytic function. As proteins, they are composed of long chains of amino acids folded into specific 3D structures. These structures form active sites that bind to specific target molecules (substrates), much like keys fit to locks. What catalysis means is the acceleration of a chemical reaction by a substance that isn’t consumed in the process. Enzymes are therefore proteins that speed up reactions and that can be reused repeatedly.
The structure of amylase enzymes allows them to target and bind to starch, and their catalytic role is to break it down into simpler sugars.
The simple sugars produced are mostly maltose, dextrins, and some glucose. These can be absorbed by microbes. Yeast and bacteria preferentially consume glucose when available, but yeast can also absorb maltose and catabolize it (another word for “break it down”) into glucose inside the cell using maltase enzymes. Some dextrins remain unfermented and contribute to the final bread’s texture and mild sweetness.
These fermentable sugars feed the yeast and bacteria, driving fermentation and flavor development. This process still occurs without enzymes, but at a much slower rate – practically, enzymes are essential.
Amylases bind to starch forming an enzyme-substrate complex, which they then cleave using water molecules in a process called hydrolysis. Adding water to flour hydrates the enzymes, unpacks them, allows them to move, and enables hydrolysis, kickstarting the whole fermentation process.
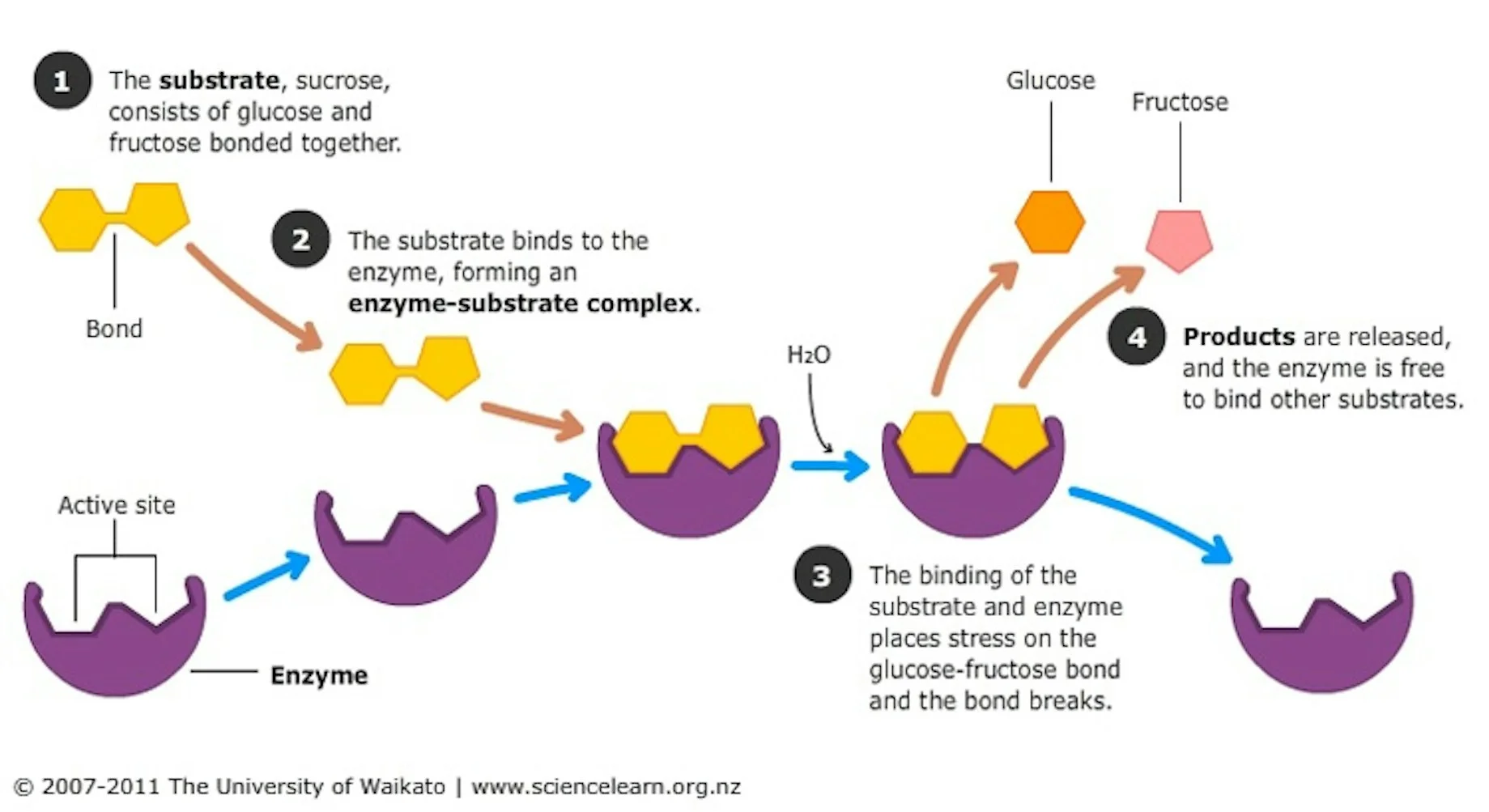
Fig 3 - Action of sucrase on sucrose (source: Science Learning Hub)
With this, we can now understand the leading dense technical phrase: amylase-catalyzed hydrolysis of starch yields fermentable carbohydrates.
While amylases work on starch, other sugars like sucrose (common table sugar) require different enzymes, like invertases (also called saccharases or sucrases). Invertases hydrolyze sucrose into glucose and fructose, that yeast and bacteria can consume. Certain yeasts, such as baker’s yeast (also called brewer’s yeast, and known in polite society as Saccharomyces cerevisiae), even naturally produce invertase when sucrose is present.
This is one reason sugar is often added to certain doughs like brioche. It’s not just for sweetness, but also because sucrose is easier and faster to break down than starch by these yeasts, which helps accelerate fermentation.
Activity of amylases in wheat increases with temperature until around 70°C, at which point amylases begin to denature (the proteins lose their 3D structure, they “unfold”). Amylases also thrive at a pH of around 5.
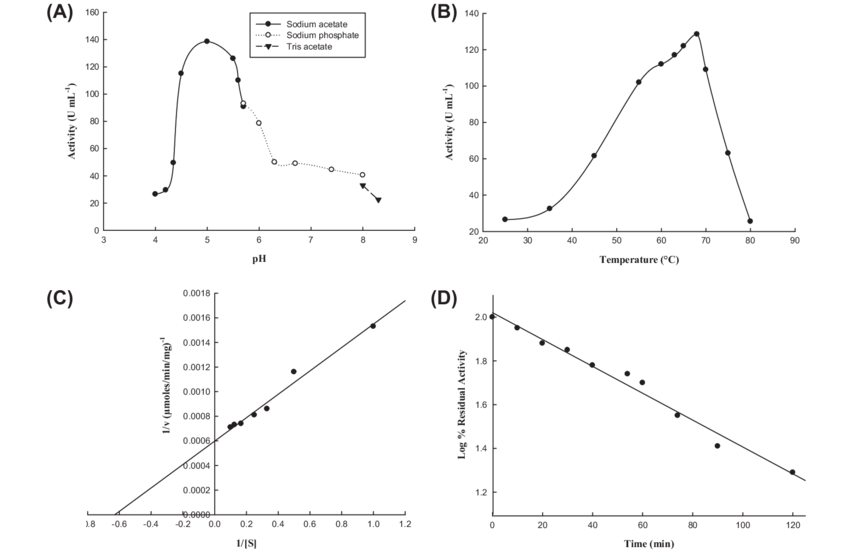
Fig 4 - Wheat α-amylase activity profile (adapted from Soni et al., 2011, ResearchGate).
Should we try to maximize their activity? In sourdough baking, the answer is no. We’re actually happy with low amylase activity, for two main reasons. First, while amylases thrive at higher temperature and a slightly acidic pH, yeasts and bacteria prefer lower temperatures and more acidic environments. Second, although fermentable sugars are essential for microbial growth, starch also plays a key role in the dough’s structure – more on that later. We need just enough amylase activity to feed the microbes, but not so much that it degrades the dough.
Certain flours contain more amylases than others. Generally, whole grain flours have higher amylase activity than refined flours. This is because a grain consists of three main parts: the bran, germ, and endosperm. The bran, the outer layer, is rich in fiber, minerals, and antioxidants. The germ, the embryo of the seed, contains fats, vitamins, and enzymes such as amylases. The endosperm, the starchy core, is composed mostly of carbohydrates and some protein. Refined flour contains only the endosperm, so limited amounts of enzymes. Notably, whole rye flour has stronger amylase activity than whole wheat flour, so you need to be a bit more careful when baking rye bread.
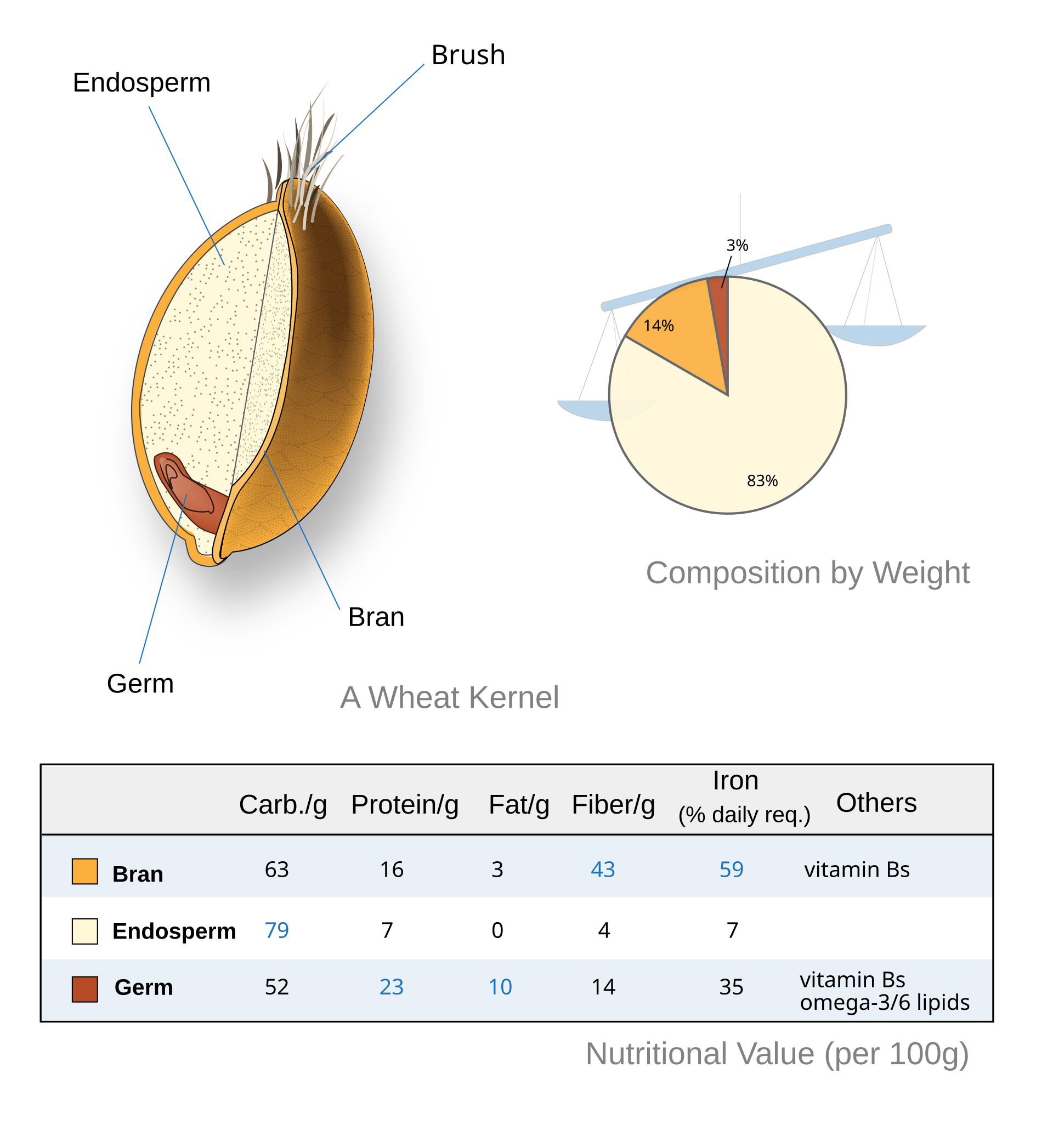
Fig 5 - Nutritional composition of a wheat kernel. Adapted from Wheat-kernel nutrition.svg, by User:ReynoldWong, CC BY-SA 4.0, via Wikimedia Commons. Original image link.
Finally, we’ve mostly focused on starch breakdown, since it’s the visible part: it produces dough rise, gas, and sourness. However, yeasts require more than just glucose to grow. They also need nitrogen compounds, released by the action of proteases (enzymes that break down protein chains), along with essential minerals, as Pasteur demonstrated in the 19th century. In addition, certain vitamins, whose vital roles were only identified later in that century, are also necessary in small amounts. Fortunately, non-refined flour offers all of this.
That’s it for today! In the next article, we’ll explore how bacterial fermentation shapes sourdough’s flavor, shelf-life, and safety.
Postscriptum: As I was writing this article, I generated a ribbon diagram of barley α-amylase. Funnily enough, just moments later, Quanta Magazine republished an article that popped up in my feed. It’s a fascinating piece about how the ribbon diagram created by Jane S. Richardson came to be: “How Colorful Ribbon Diagrams Became the Face of Proteins”.
